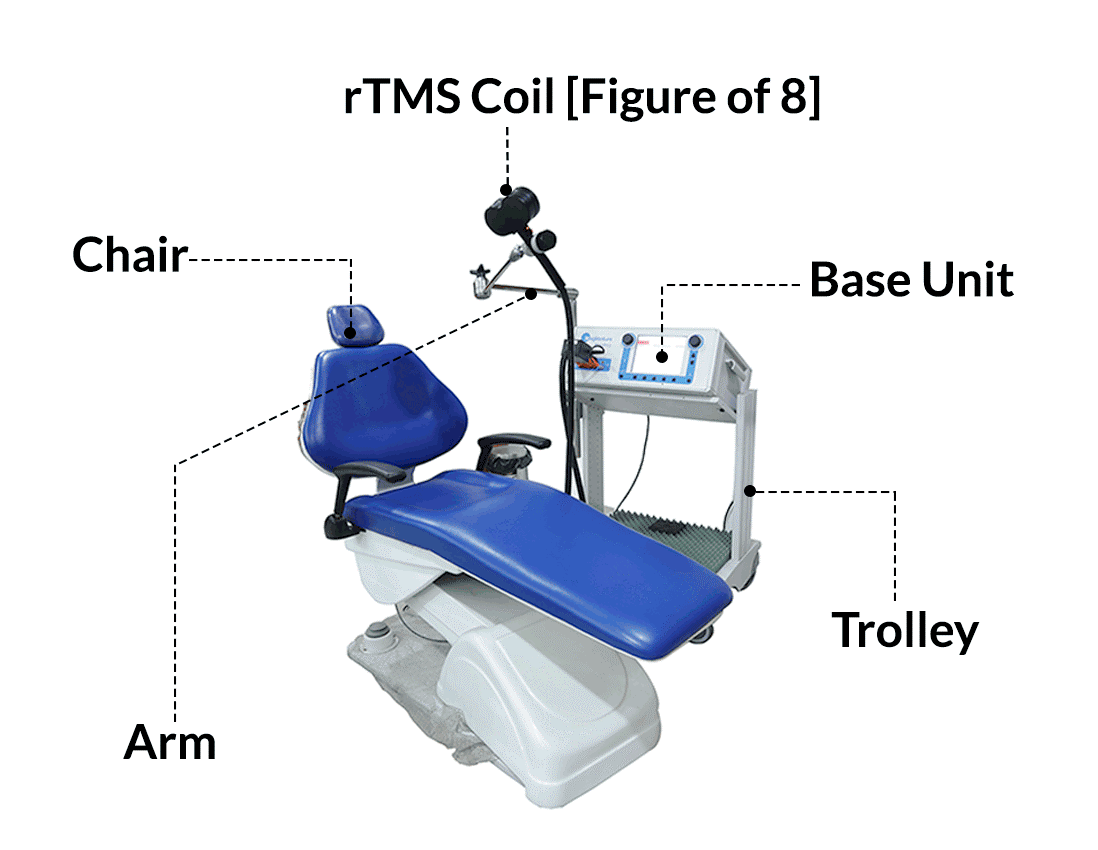
REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION(rTMS)
rTMS stands for “repetitive transcranial magnetic stimulation.” rTMS is a non-invasive FDA-cleared medical procedure for the treatment of depression in adults. rTMS is a brain stimulation technique that depends on the generation of brief magnetic fields using an insulated coil that is placed over the scalp.
These magnetic fields are the same type and strength as those used in magnetic resonance imaging (MRI) machines. The magnetic pulses generate a weak electrical impulses in the brain that briefly activates neural circuits at the stimulation site. rTMS has been shown to be a safe and well-tolerated procedure that can be an effective treatment for adult patients with depression who have not benefitted from antidepressant treatment.
HOW DOES rTMS WORK?
During an rTMS session, a magnetic coil is placed against your scalp near your forehead. The magnet painlessly delivers a pulse that stimulates nerve cells in the region of your brain involved in mood control and depression. It’s activates the regions of the brain that have decreased activity in depression.

WHAT ILLNESSES HAS rTMS BEEN SUCCESSFUL IN TREATING?
The FDA approved rTMS to treat Treatment Resistant Depression in 2007.
Since then, rTMS has been used to treat a variety of psychiatric and neurological disorders with mixed results.
HOW EFFECTIVE IS rTMS IN TREATING DEPRESSION?
rTMS is used to treat very severe cases of depression, especially those patients who do not adequately respond well to medications, or those in whom high dose medications are not usually well tolerated.
rTMS was approved by the FDA for the treatment of Resistant Depression in 2007 and has been used extensively all over the world since.
- In a study of 1383 patients, active treatment with rTMS was concluded to be better than Sham treatment.1
- In a pooled sample size of 230 patients, 47% patients achieved >50% reduction in symptoms when rTMS was added to the existing medication regimen.2
- Also, early initiation of rTMS along with medicines seems to be more effective than just prescribing medicines alone.
- An analysis of 213 patients revealed that when rTMS is combined with medicines, early in the illness, patients are “TWICE MORE” likely to achieve complete resolution of their symptoms, then those who are just on medication alone. (odds ratio 2.4, 95% CI 1.3-4.6) 3
QUICK FACTS ABOUT rTMS
How many sessions are required to be taken for Depression?
For Medical Treatment Resistant Depression: 40 Sessions
For Depression Not Resistant to Medical Treatment: 20-30 Sessions
(Note: This will depend on the clinical status and response to medications)
Duration of a Session: 30 minutes approximately Two such sessions can be given simultaneously.
When can I start seeing the response to treatment?
Response is usually seen after 10-15 sessions are completed. Patients start feeling distinct changes in mood and energy levels.
In cases of accelerated rTMS, positive changes from treatment can continue up to 1 month post the delivery of rTMS.
How long do the effects last?
In our clinical experience, patients who undergo adequate rTMS treatment, maintain the therapeutic effect for >1-2 years.
All patients who are started on rTMS need to continue with medications as prescribed before the start of rTMS treatment.
Accelerated TMS
A form of treatment where such 4 sessions or 12000 pulses/day can be given safely.
With accelerated TMS, patients only need 10 days to complete the entire course of treatment.
rTMS is a procedure carried out on OPD basis and doesn’t require patient to be admitted in the hospital. There are no post-procedure complications or side effects and hence patient can go home after the session.
What happens if symptoms re-appear post rTMS?
Symptoms can re-appear post an rTMS course, under two conditions:
Can rTMS be given if the patient is already on medicine?
Yes. This treatment will not interfere adversely with medication. Rather, both will have a complimentary effect on the underlying disease process.
HOW IS rTMS COMPARABLE TO ELECTROCONVULSIVE THERAPY (ECT)?
To understand the answer to this question, one must understand some other aspects.
| Mild-moderate depression | Improve treatment response at early stages of illness |
| No stigma attached | No memory issues |
| Non-invasive, minimal-no side effect | Safe in patients with other physical conditions such as cardiac patients, renal patients, liver damage patients |
PREGNANCY: NO SIDE-EFFECT TO MOTHER/BABY. ABSOLUTELY SAFE
| Melancholic Depression Depression with Psychosis | Previous history of good response to ECT |
| Aggression/ Severe Irritability | Complete social isolation/ poor functioning |
| Significant feeling of hopelessness/ worthlessness | Severely reduced appetite |
| Severe slowing of actions and thoughts | Residual symptoms not responding to medications |
SUICIDAL THOUGHT/ACTIONS/ATTEMPTS
WHAT PROBLEMS DO PATIENTS COMMONLY REPORT?
The main advantage that rTMS provides over other forms of brain stimulation techniques is its safety.
Most commonly experienced:

Headache

Heaviness of head

Tingling numbness of scalp

Dizziness
All these side effects are self-limiting. Some patients need to consume an over the counter analgesic for the resolution of headache (e.g. Ibuprofen). The most serious side effect with rTMS treatment is the possibility of a seizure occurrence. The probability of a seizure is ∼0.01% to 0.1%
| rTMS | 0.01% to 0.1% |
| Anti-Depressant Medicines | 0.1% to 0.6% |
| Normal Population | 0.07% to 0.09% (Chances of spontaneous seizure generation) |
| Anti-Depressant Medicines | History of Seizure Episodes/
Epilepsy or Brain Damage Metallic implants in the brain |
| Normal Population | Cardiac Pacemakers Implantable Defibrillators |
FAQs ON rTMS
Currently available data from repeated application of high intensity, time-varying magnetic fields to humans, as in magnetic resonance imaging, do not suggest that the long-term risks of rTMS are significant.
Many patients with schizophrenia experience auditory hallucinations. 25% of patients with schizophrenia have treatment resistant hallucinations. rTMS targeted over the auditory cortex has the potential to significantly reduce the occurrence of voices. Also, rTMS can be used to improve motivation, mood and memory of patients with schizophrenia.
Post-Stroke depression is very common in patients who have suffered a stroke recently. A course of rTMS, using the same depression protocol, can significantly reduce the severity of depression and improve functioning.
Patients report a measurable reduction in pain after rTMS. It has a selective effect by increasing pain tolerance & altering pain processing, thereby providing long-lasting pain relief.
Yes. It has been used for its role in preventing the Migraine attacks from taking place and can reduce the frequency & severity of the Migraine headache.
REFERENCES
- Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010; 71:873.
- Liu B, Zhang Y, Zhang L, Li L. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry 2014; 14:342.
- Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry 2013; 74:e122

Consultation Timing
Monday - Saturday
Morning: 9:00 -1:00 PM
Evening: 4:00 - 7:00 PM
Follow Us
Get In Touch
Parth Hospital,
3rd Floor Sigma Excellence,
Near Management Enclave,
Opposite Falguni Gruh Udyog,
Ahmedabad, 380015
For Appointment

